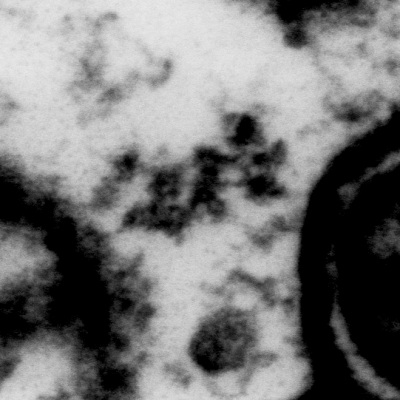
3D Electron Microscopy
Synapses connect neurons into networks, creating the physical substrate for information processing in the brain. The human brain, which weighs only about three pounds, contains hundreds of trillions of synapses. Because synapses are so small – the synaptic cleft is only 20 nanometers across – they can only be directly visualized using an electron microscope. We use serial electron microscopy to reconstruct volumes of brain tissue in three dimensions at nanometer resolution. Especially in combination with neuroanatomical tracing and immunohistochemical identification of cells, this powerful approach not only allows us to quantify and detect changes in synapses and connectivity that occur when memories are formed, it also gives us a uniquely wholistic and intimate view of the microscale architecture of the brain.
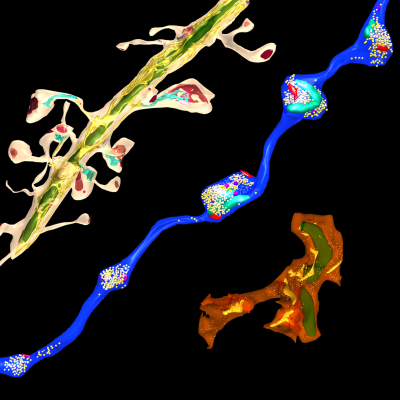
Local Protein Synthesis
Regulated changes in gene expression have long been known to play a central role in learning and memory. Protein synthesis occurs in neuronal processes, where it can serve as a rapid source of proteins near activated synapses. We are interested in the role of local protein synthesis at synapses in forming and maintaining memories, and more broadly in what decentralized control of gene expression within neurons can tell us about how information is stored in brain circuits. Contrary to longstanding assumptions, we have found that long-range cortical axons projecting to the amygdala in adult animals contain a diverse assortment of mRNAs, and that their mRNA content is regulated by learning. The heterogeneous distribution of mRNAs throughout the brain is recognized as a fundamental feature of brain structure, and individual brain areas and neuronal cell types may be characterized by particular gene expression patterns. Protein synthesis in distal axons means that some genes may be transcribed in one brain area and translated in another, meaning that regulation of gene expression is not cell-specific, but rather circuit-specific or synapse-specific. To investigate the dynamics of local translation, we use a combination of electron microscopy labeling techniques, viral vector delivery of affinity tagged ribosomes, and next generation sequencing methods.
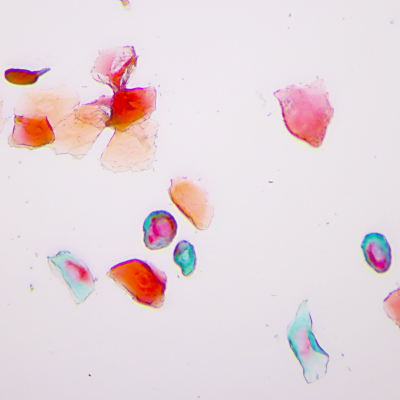
Synaptic Circuitry in the Female Brain
As in most biomedical science, female subjects have historically been left out of neurobiology research. In the realm of fear and anxiety research, sexual dimorphisms in behaviors and suspected variability over the reproductive cycle have justified the predominant use of male subjects. If these factors are indeed significant, they represent not merely experimental confounds but valuable, unexplored opportunities to gain a deeper understanding of brain function. If fear and anxiety manifest differently in females and males, studying the brain substrates that underlie these differences could illuminate fundamental brain mechanisms of emotion regulation. Likewise, substantial behavior changes over the course of the reproductive cycle offer an opportunity to observe the dynamic range of brain circuit reorganization during regulation of innate, adaptive anxiety states. We incorporate female subjects into our work and use multiple strategies, such as measurement of serum hormone levels and expanded behavioral analysis, to enhance our understanding of what is, and what is not, different between males and females.